 https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
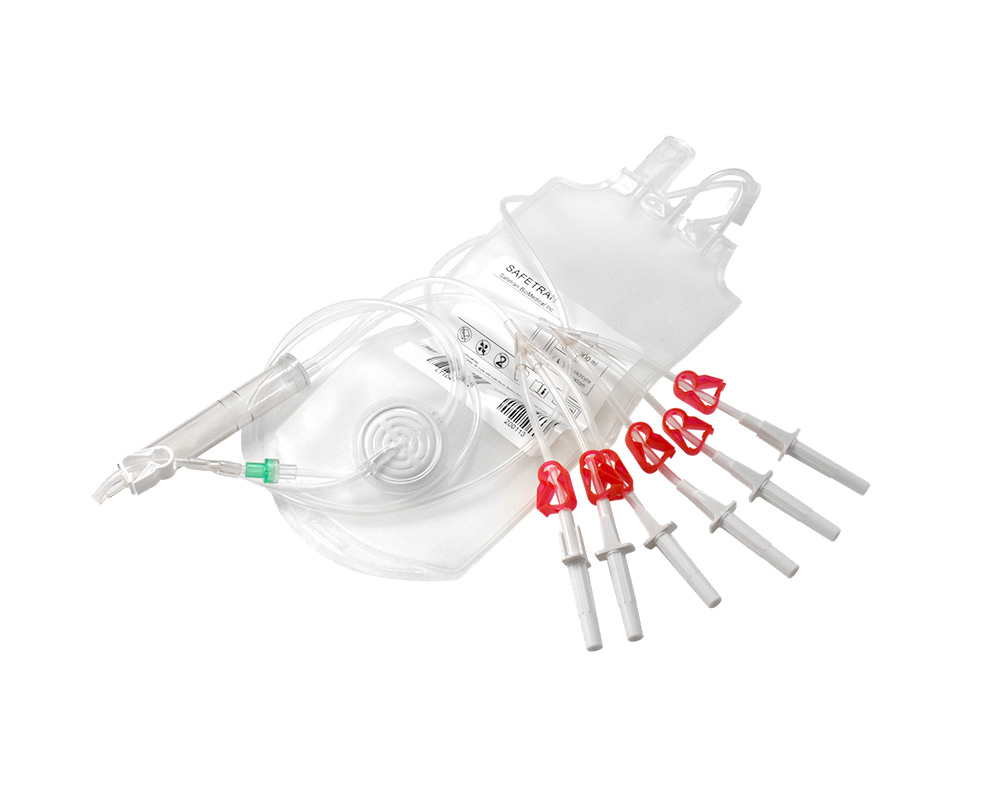
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器
https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器服務項目
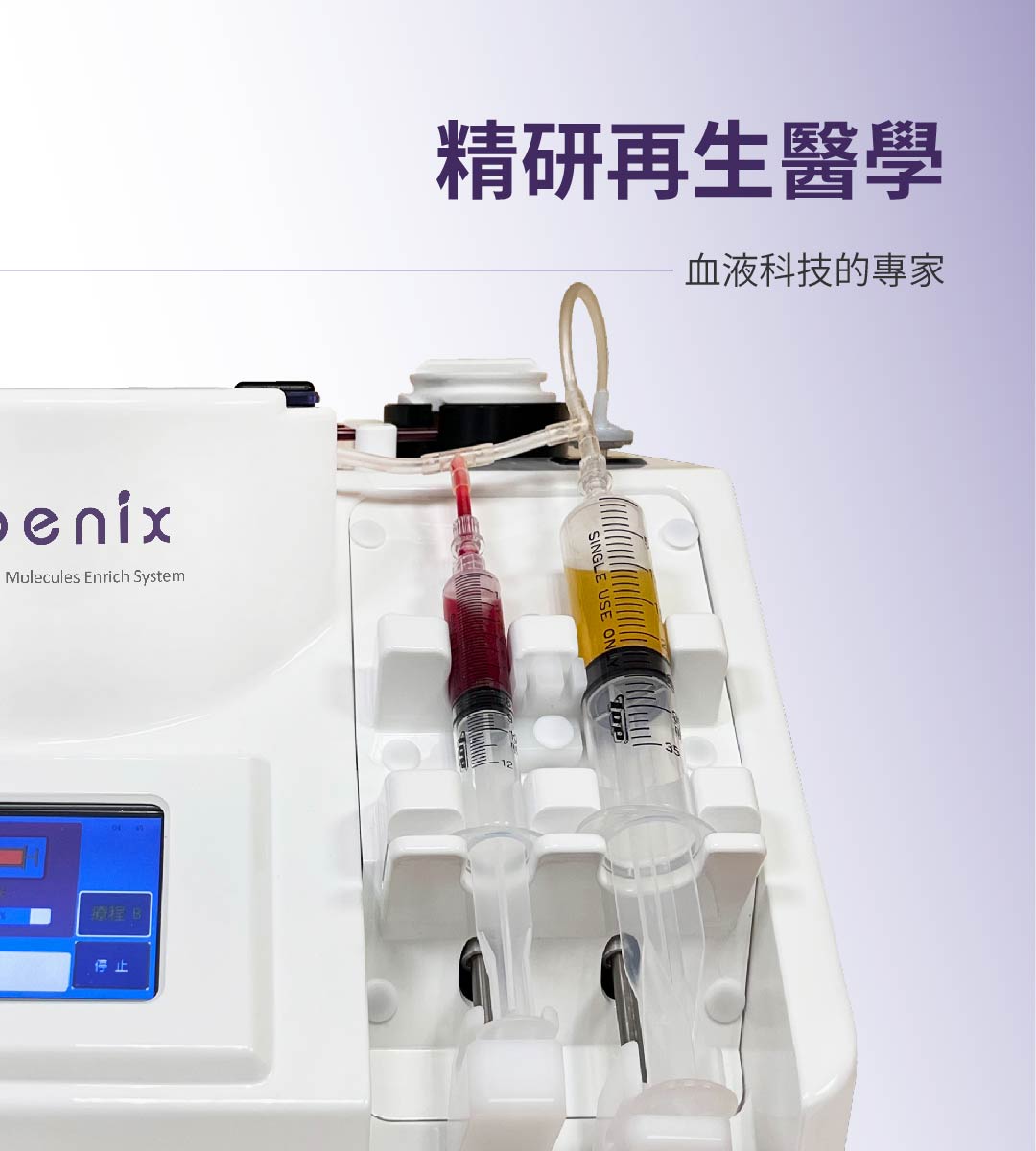
再生醫學
因子療法(Autologous Bioactive Molecules,BαM),為傳統PRP療法更精準的名稱,富含血小板血漿(Platelet-Rich Plasma)在臨床應用至今已經逾30年…
產品簡介
 https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器
https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器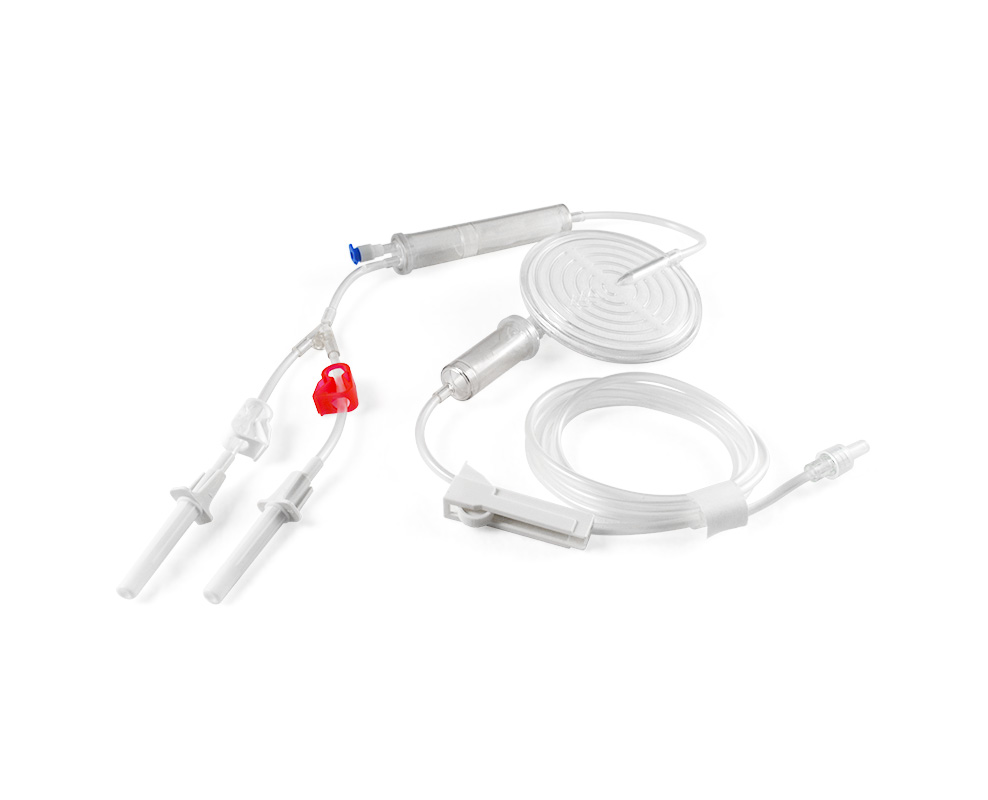 https://safetran.com.tw/wp-content/uploads/2021/10/LR5022_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-05 15:30:512023-12-01 15:13:21全血/紅血球專用過濾器
https://safetran.com.tw/wp-content/uploads/2021/10/LR5022_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-05 15:30:512023-12-01 15:13:21全血/紅血球專用過濾器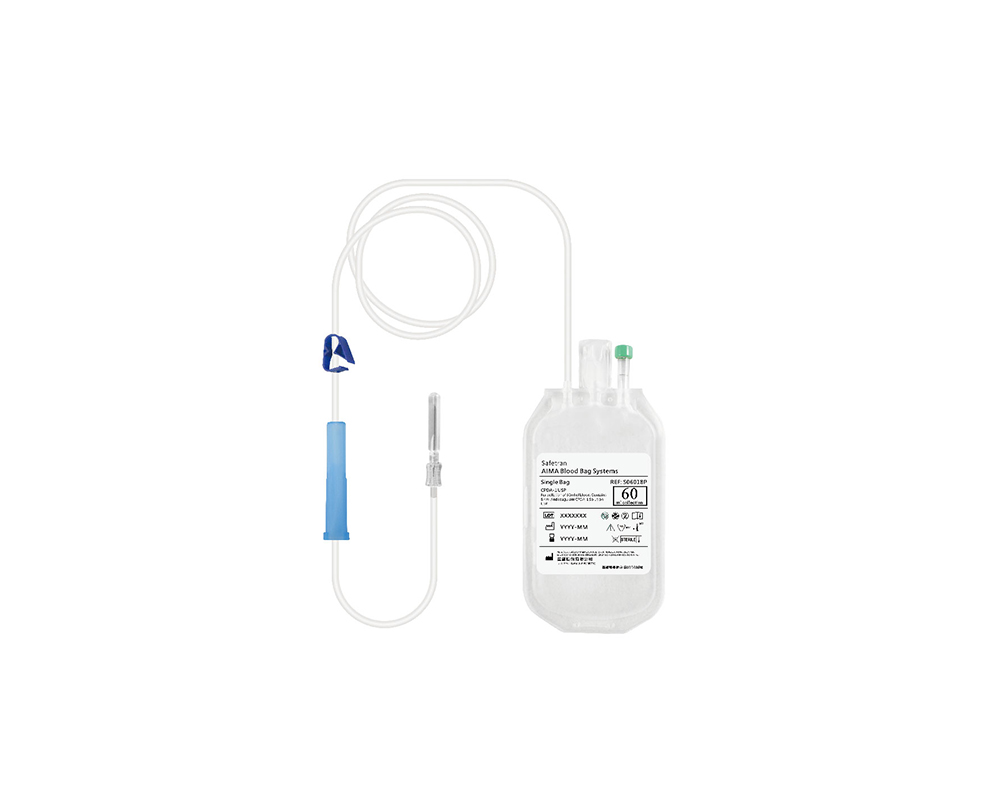 https://safetran.com.tw/wp-content/uploads/2021/10/bloodbag_845_684_S060-07.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 17:00:192024-03-12 11:12:44血袋系統
https://safetran.com.tw/wp-content/uploads/2021/10/bloodbag_845_684_S060-07.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 17:00:192024-03-12 11:12:44血袋系統 https://safetran.com.tw/wp-content/uploads/2021/10/lr_filter_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 16:55:192023-12-01 17:15:44白血球過濾器
https://safetran.com.tw/wp-content/uploads/2021/10/lr_filter_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 16:55:192023-12-01 17:15:44白血球過濾器 https://safetran.com.tw/wp-content/uploads/2021/10/RHEA_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-26 11:58:522023-11-23 17:45:49Rhea全自動生物活性因子系統
https://safetran.com.tw/wp-content/uploads/2021/10/RHEA_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-26 11:58:522023-11-23 17:45:49Rhea全自動生物活性因子系統產品簡介
 https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器
https://safetran.com.tw/wp-content/uploads/2023/09/LT1043_845_684-1.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-06 10:22:222023-12-01 15:17:09血小板專用過濾器 https://safetran.com.tw/wp-content/uploads/2021/10/LR5022_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-05 15:30:512023-12-01 15:13:21全血/紅血球專用過濾器
https://safetran.com.tw/wp-content/uploads/2021/10/LR5022_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2023-09-05 15:30:512023-12-01 15:13:21全血/紅血球專用過濾器 https://safetran.com.tw/wp-content/uploads/2021/10/bloodbag_845_684_S060-07.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 17:00:192024-03-12 11:12:44血袋系統
https://safetran.com.tw/wp-content/uploads/2021/10/bloodbag_845_684_S060-07.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 17:00:192024-03-12 11:12:44血袋系統 https://safetran.com.tw/wp-content/uploads/2021/10/lr_filter_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 16:55:192023-12-01 17:15:44白血球過濾器
https://safetran.com.tw/wp-content/uploads/2021/10/lr_filter_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-27 16:55:192023-12-01 17:15:44白血球過濾器 https://safetran.com.tw/wp-content/uploads/2021/10/RHEA_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-26 11:58:522023-11-23 17:45:49Rhea全自動生物活性因子系統
https://safetran.com.tw/wp-content/uploads/2021/10/RHEA_845_684.jpg
800
988
safetranadmin
https://safetran.com.tw/wp-content/uploads/2023/09/safetranlogo_01-1-300x138.png
safetranadmin2021-10-26 11:58:522023-11-23 17:45:49Rhea全自動生物活性因子系統最新消息
最新消息

Safetran is participating ISBT Gothenburg
Safetran is participating ISBT Gothenburg this year!
We are presenting our brand-new “Eco-Friendly Surface Modification Technology”,This processing method is used on our “Leukocyte Reduction ......
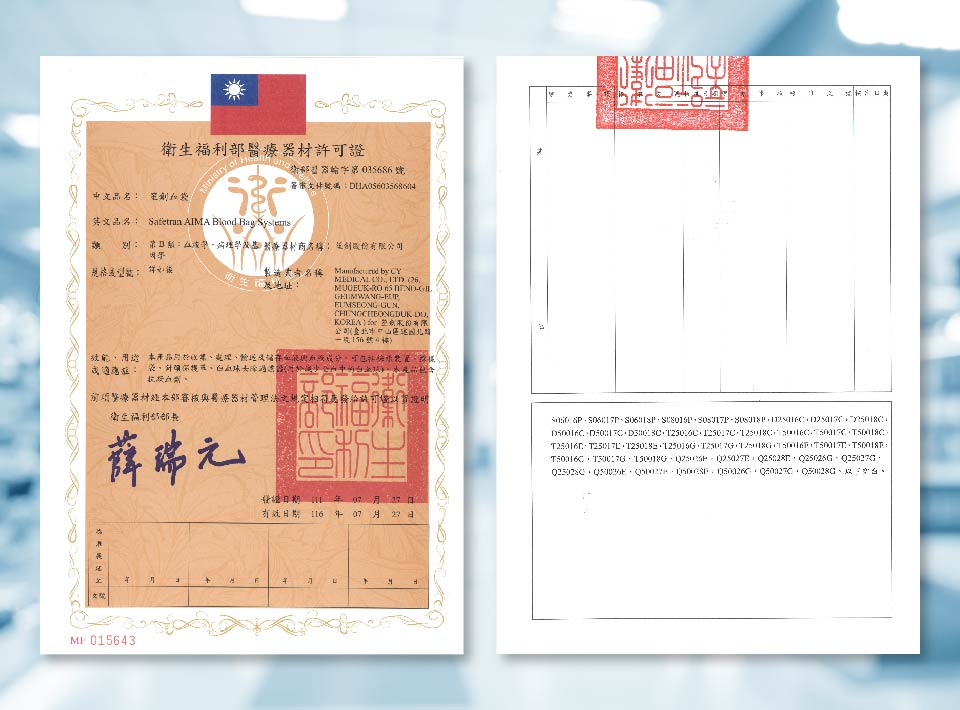
笙創血袋取得台灣許可證
笙創血袋 取得台灣衛生福利部許可證
證號: 衛部醫器輸字第035686號


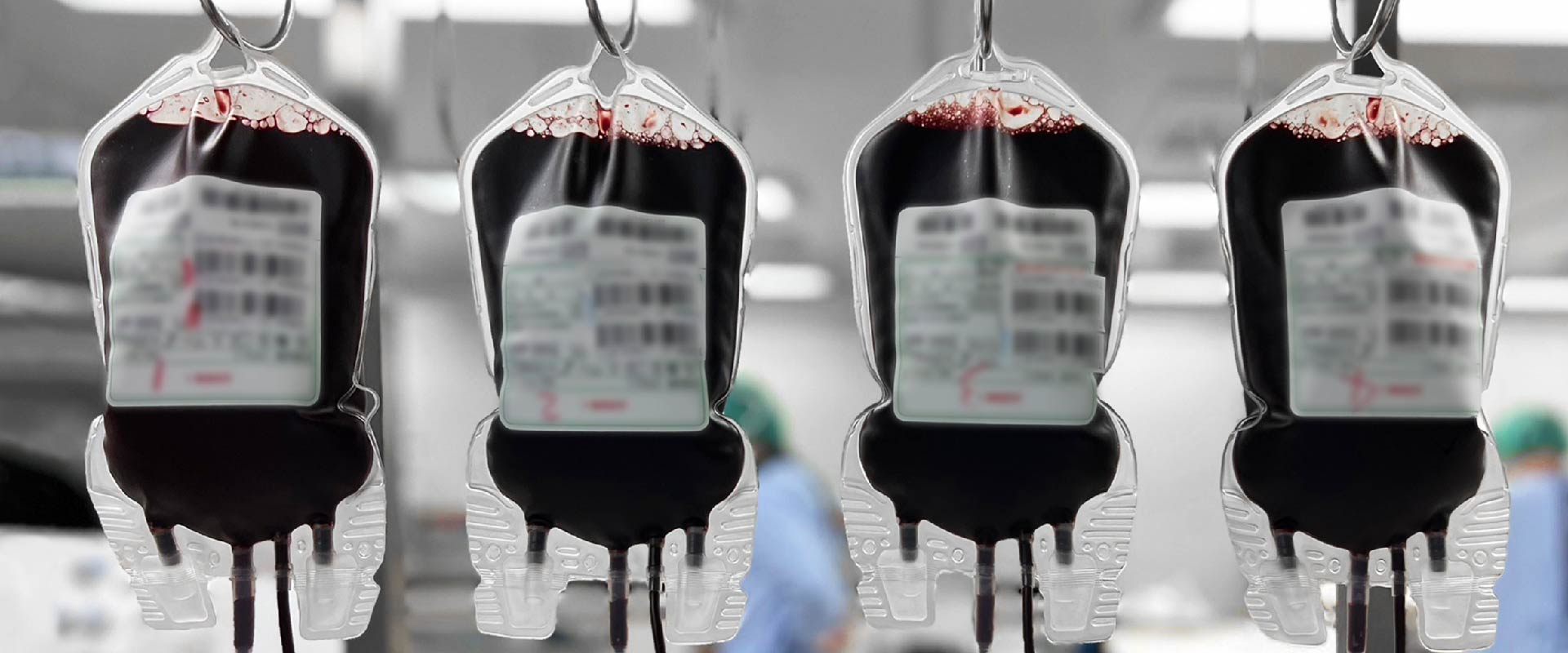




Safetran is participating ISBT Gothenburg
/在: 最新消息 /通過: safetranadminSafetran is participating ISBT Gothenburg this year!
We are presenting our brand-new “Eco-Friendly Surface Modification Technology”,This processing method is used on our “Leukocyte Reduction ……
笙創血袋取得台灣許可證
/在: 最新消息 /通過: safetranadmin笙創血袋 取得台灣衛生福利部許可證
證號: 衛部醫器輸字第035686號